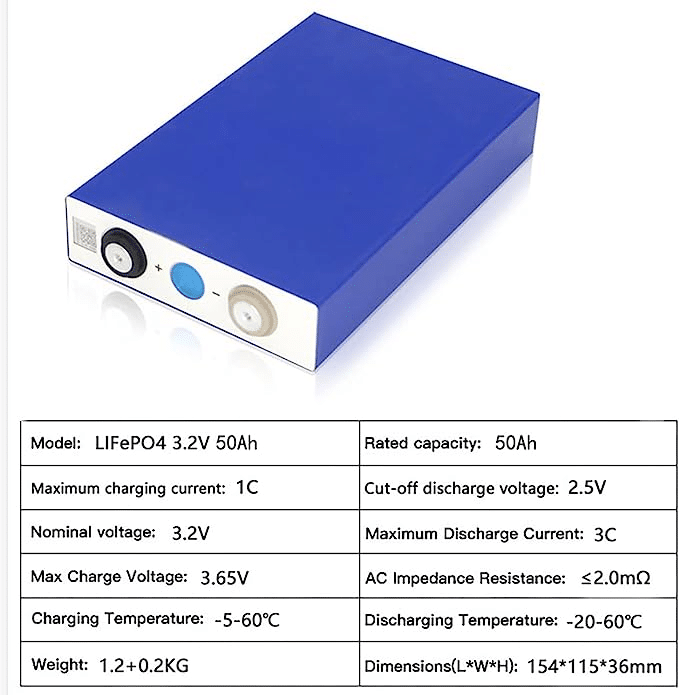
Lithium-ion battery safety is an intricate matter. One major threat lies within random internal short circuiting that occurs within batteries resulting in on-site failure and uncontrolled heating; hence the development and use of materials with superior thermal stability should be prioritized in efforts to further increase future lithium-ion safety performance.
Improving the thermal stability of battery materials
Synthesis techniques that increase thermal stability include optimising conditions and methods; synthesizing materials with excellent thermal stability is also possible, or alternatively doping and coating techniques may be employed to achieve it.
Thermal stability of negative electrode materials depends on three elements: type of material used to form negative electrodes, particle size distribution and SEI film formation stability. By mixing particles from various sizes together in equal proportions as negative electrodes, one can achieve expansion of contact areas between particles, reduced impedance levels, higher capacity and decreased likelihood of active metal lithium precipitation.
SEI film formation quality directly affects charging and discharging performance and safety in lithium-ion batteries. Modifying carbon surface through weak oxidizing, reducing, doping or doping-modified doping or using spherical or fibrous carbon materials are all beneficial in improving SEI film quality.
Stability of electrolyte depends upon its composition: lithium salt and solvent selection can have a dramatic impact on its stability. Utilizing lithium salts with strong thermal stability and solvents with wide potential stabilization windows will increase thermal stability; adding high boiling point solvents or those without flash points as part of an electrolyte solution can further bolster this effect and ensure its safety.
The types and quantities of conductive agents and binders also play a part in affecting battery thermal stability. Binders react with lithium at high temperatures to produce large amounts of heat; different binder types produce differing amounts; PVDF produces nearly twice as much heat than fluorine-free biners, so replacing PVDF with these alternative binders could improve thermal stability significantly.
There are currently 112 members. We thank them all for being part of our community!
Enhancing battery overcharging protection
To avoid overcharging of lithium-ion batteries, dedicated charging circuits or safety valves may be installed onto individual batteries in order to monitor and control charging/discharging process; secondly, positive temperature coefficient resistors (PTCs), with their mechanism of increasing internal resistance when overcharged batteries heat up due to overcharging; special separators can also be utilized when abnormalities cause temperatures of separator to become excessive; this prevents lithium ions migrating out and overcharging of battery; PTCs prevent this through increasing resistance which causes internal resistance increase thus limiting overcharging current; finally special separators come into use when abnormalities cause excessive temperatures of separator and block pores within it, thus stopping migration while protecting it against overcharging current;
Avoiding short circuits in batteries
A typical separator’s porosity should range between 40-50% and its distribution is uniform. A separator with pores of 10nm may help block movement of extremely small particles that might otherwise enter batteries; thus improving their safety.
Diaphragm insulation voltage relates directly to its ability to prevent contact between positive and negative electrodes of a battery, with material, structure and assembly conditions all having an influence.
Use of composite membranes such as PP/PE/PP with a significant difference between their thermal closure temperature and melting temperature can help prevent battery thermal runaway. Coating diaphragm surfaces with ceramic layers is one way to increase temperature resistance; low melting point PE (125), used in combination with high mechanical strength separators like PP (155), can ensure closed cell effect at lower temperatures while providing protection from positive/negative electrode contact, providing safety to battery use.
Everyone knows that replacing metal lithium negative electrodes with graphite ones can transform the deposition and dissolution of lithium on negative electrode surfaces during charging and discharging into its incorporation and detachment in carbon particles, thus avoiding dendrite formation. But this doesn’t guarantee complete battery safety; during charging processes too high positive electrode capacities could deposit metal lithium onto negative electrodes; while too high negative electrode capacities would lead to severe capacity losses in battery capacity loss.
Coating thickness and uniformity also have an impactful influence on how lithium ions enter or exit an active substance. If, for instance, density on a negative electrode surface is thick and uneven during charging processes, then its polarization size may fluctuate during charging phases leading to depositions of metallic lithium at certain locations on its surface.
Unsuitable usage conditions can also contribute to short circuits in batteries. At low temperatures, due to an excess deposition rate of lithium ions being greater than their insertion rate, metal lithium deposits on electrode surfaces and causes short circuits. Therefore, controlling proportions between positive and negative electrode materials while improving uniformity coating is key to avoiding lithium dendrite formation.
Crystallization of adhesives and the formation of copper dendrites are two additional causes for internal short circuits in batteries. When coating, all solvents in the slurry are removed by coating and baking heating – but if that heating temperature becomes excessively hot, adhesive crystallization could occur, leading to active substance peel-off that results in internal short circuits within batteries.
Under over discharge conditions, when the battery reaches 1-2V after over discharging it will start dissolving and precipitating on the positive electrode. When discharging below 1V copper dendrites will form on its surface causing internal short circuits in lithium-ion battery cells resulting in short circuits being established within it.